How Generative AI Is Revolutionizing the Pharma Industry
Table of Contents
Are you working in pharmaceutical development and feeling overwhelmed by pressure to deliver breakthrough treatments faster than ever? Have you wondered how your organization can cut drug discovery timelines from decades to years while maintaining safety standards? Generative artificial intelligence is already transforming how leading pharmaceutical companies approach everything from molecular design to clinical trials.
The pharmaceutical industry faces unprecedented challenges today. You likely know the sobering statistics: it takes 10-15 years and costs over $2.6 billion to bring one drug to market. Meanwhile, your patients cannot wait, regulatory requirements grow complex, and competition intensifies globally. Here is where generative AI in pharma becomes your strategic advantage.
By the end of this comprehensive guide, you will understand exactly how generative AI can revolutionize your pharmaceutical operations. We will explore practical applications you can implement, address challenges you might face, and provide a clear roadmap for adoption. Let us dive into this transformative technology reshaping pharmaceutical innovation.
Key Takeaways
- Readers will be able to understand how generative AI is transforming pharmaceutical drug discovery, development, and clinical operations end to end.
- Recognize why the pharma industry is rapidly adopting generative AI to reduce timelines, lower costs, and improve R&D success rates.
- Identify high-impact use cases for generative AI across drug discovery, clinical trials, manufacturing, and regulatory documentation.
- Evaluate the tangible benefits of generative AI, including faster time-to-market, improved decision-making, and enhanced innovation capacity.
- Understand the key challenges, regulatory considerations, and ethical requirements involved in adopting generative AI in pharma.
- Apply a clear, step-by-step framework for implementing generative AI strategies with the right data foundations, governance, and expert partners.
Table of Contents
Understanding Generative AI in the Pharmaceutical Industry
What exactly makes generative AI different from traditional artificial intelligence tools you might already use in pharmaceutical work? Unlike conventional AI systems that analyze existing data to find patterns, generative AI creates entirely new content and solutions.
In your pharmaceutical context, this means the technology can generate novel molecular structures, design new drug compounds, create optimized clinical trial protocols, and produce regulatory documentation. Think of it as having a brilliant research partner who never sleeps and explores millions of possibilities simultaneously.
Generative AI in pharma operates through sophisticated neural networks trained on vast datasets of molecular information, clinical data, and research literature. When you input specific parameters or requirements, the system generates multiple novel solutions that meet your criteria. This capability transforms how you approach drug discovery, moving from reactive analysis to proactive creation.
The technology excels in areas where traditional methods struggle. Can you imagine designing thousands of potential drug candidates in hours rather than months? Generative models make this possible by understanding underlying patterns in successful pharmaceuticals and creating new variations with desired properties.
For your organization, this represents a fundamental shift from hypothesis-driven research to AI-augmented discovery. You maintain scientific oversight and decision-making authority while leveraging AI to exponentially expand your creative and analytical capabilities.
Why the Pharma Industry Is Adopting Generative AI
Have you calculated how much your organization spends on failed drug candidates? The pharmaceutical industry faces harsh reality: approximately 90% of drug candidates fail during development, often after years of investment and research.
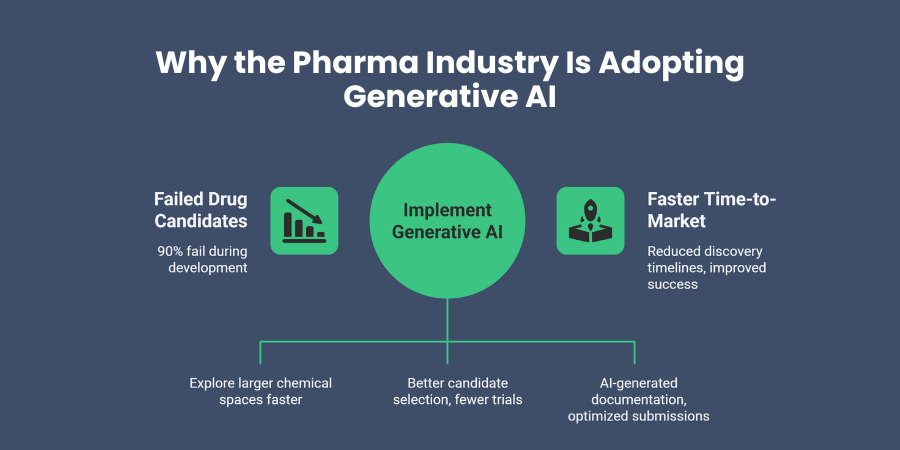
Your industry confronts several critical challenges that generative AI directly addresses. First, lengthy drug discovery cycles strain your resources and delay patient access to treatments. Traditional methods require extensive laboratory work, animal testing, and multiple iteration cycles that consume precious time.
Second, you deal with increasingly complex regulatory requirements across multiple markets. Each submission demands comprehensive documentation, safety data, and efficacy proof that traditional approaches struggle to optimize efficiently.
Third, the explosion of biomedical data creates both opportunities and overwhelming complexity. Your researchers cannot manually process millions of research papers, clinical datasets, and molecular databases published annually.
Generative AI in pharma addresses these pain points systematically. The technology accelerates hypothesis generation, reduces experimental costs through better candidate selection, and helps you navigate regulatory complexity with AI-generated documentation.
Consider the competitive advantage this creates for your organization. While competitors rely on traditional methods, you can explore vastly larger chemical spaces, optimize clinical trial designs, and accelerate time-to-market for breakthrough treatments.
The adoption momentum is undeniable. Leading pharmaceutical companies report 40-60% reductions in early-stage discovery timelines and significant improvements in candidate success rates when implementing generative AI strategies.
Key Use Cases of Generative AI in Pharma
Where can you apply generative AI most effectively within your pharmaceutical operations? The applications span your entire value chain, from initial research through commercial launch.
In drug discovery, generative models excel at molecular design and optimization. You can input desired therapeutic properties, and the AI generates novel compounds with those characteristics. This approach has already produced several compounds currently in clinical trials.
Target identification represents another powerful application. When you suspect a biological pathway contributes to disease but lack clear targets, generative AI can analyze complex biological networks and suggest novel intervention points.
Clinical trial optimization offers immediate practical benefits for your operations. Generative AI can design trial protocols, optimize patient inclusion criteria, predict enrollment challenges, and generate synthetic control groups for certain studies.
Personalized medicine applications help you develop treatments tailored to specific patient populations. By analyzing genetic, clinical, and lifestyle data, generative models can predict individual treatment responses and suggest personalized dosing regimens.
Manufacturing optimization through AI-generated process improvements can reduce costs and improve quality. The technology analyzes production data to suggest equipment settings, predict maintenance needs, and optimize batch processes.
Regulatory documentation generation streamlines your compliance processes. Generative AI can draft regulatory submissions, create safety reports, and ensure consistency across multiple regulatory jurisdictions.
How Generative AI Is Transforming Drug Discovery and Development
Are you ready to see how generative AI fundamentally changes your approach to drug discovery? The transformation begins with how you identify and optimize lead compounds.
Traditionally, your discovery teams might screen thousands of existing compounds hoping to find promising candidates. Generative AI flips this process by creating novel compounds designed specifically for your target. Instead of searching through existing chemical libraries, you generate entirely new molecular structures with desired properties.
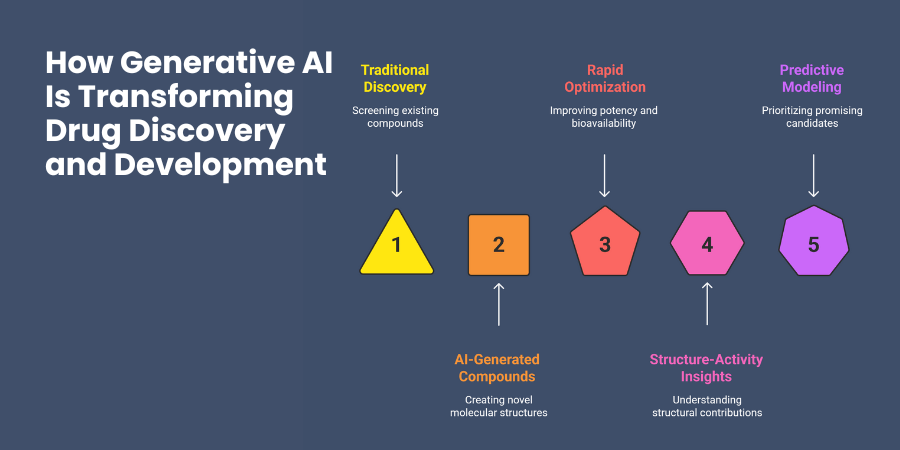
The speed improvement is remarkable. What previously required months of laboratory synthesis and testing can now happen computationally in days or weeks. You can explore chemical spaces that would be impossible to investigate through traditional high-throughput screening.
Lead optimization becomes more systematic and predictable. When you identify a promising compound with some limitations, generative models can suggest structural modifications to improve potency, reduce toxicity, or enhance bioavailability.
The technology also improves your understanding of structure-activity relationships. As generative models create and evaluate thousands of molecular variations, they reveal patterns about which structural features contribute to desired therapeutic effects.
Predictive modeling capabilities help you make better go/no-go decisions earlier in development. By generating comprehensive safety and efficacy predictions, you can prioritize the most promising candidates and avoid costly late-stage failures.
Generative AI in Clinical Trials and Research Operations
How can generative AI revolutionize your clinical trial operations and improve success rates? The applications extend far beyond simple data analysis into active trial design and optimization.
Protocol generation represents a game-changing capability for your clinical operations. Generative AI can analyze successful trial designs, regulatory requirements, and your specific therapeutic area to create optimized protocols that maximize success probability.
Patient recruitment optimization addresses one of your most persistent challenges. By analyzing demographic data, disease prevalence, and historical enrollment patterns, generative models can predict recruitment feasibility and suggest site selection strategies.
Synthetic control group generation offers exciting possibilities for rare disease studies where traditional control groups are difficult to establish. The technology can create statistically valid control populations based on historical data and disease progression models.
Real-time trial optimization allows you to adapt protocols based on emerging data. Generative AI can suggest protocol amendments, dosing adjustments, or endpoint modifications that improve trial outcomes without compromising regulatory compliance.
Data synthesis and analysis capabilities help you extract maximum value from clinical datasets. The technology can identify subtle patterns, predict patient responses, and generate insights that inform future trial designs.
Benefits of Generative AI in Pharma
What specific advantages can you expect when implementing generative AI in your pharmaceutical operations? The benefits span operational efficiency, scientific capability, and business performance.
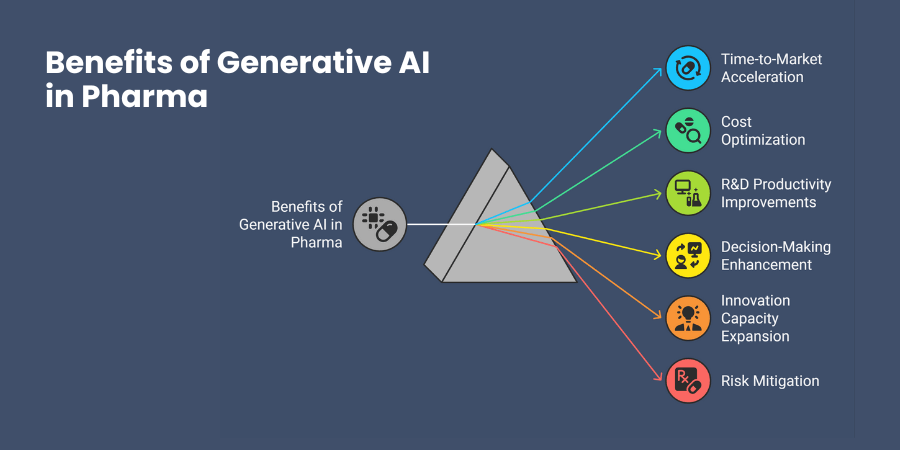
Time-to-market acceleration represents the most visible benefit for your organization. Companies using generative AI report 30-50% reductions in discovery timelines and 20-30% faster clinical development cycles. This speed advantage translates directly into competitive positioning and earlier revenue generation.
Cost optimization occurs throughout your development pipeline. By improving candidate selection accuracy, you reduce expensive late-stage failures. Better trial designs minimize patient recruitment costs and protocol deviations. Automated documentation generation reduces regulatory preparation expenses.
R&D productivity improvements help you accomplish more with existing resources. Your researchers can explore larger chemical spaces, test more hypotheses, and generate higher-quality candidates without proportional increases in laboratory costs.
Decision-making enhancement comes from better predictive capabilities. When you can accurately forecast compound behavior, trial outcomes, and regulatory responses, you make more informed strategic decisions that improve overall portfolio performance.
Innovation capacity expansion allows you to pursue previously impossible research directions. Generative AI in pharma enables exploration of novel chemical spaces, unconventional therapeutic approaches, and personalized medicine strategies that traditional methods cannot address efficiently.
Risk mitigation through better prediction and optimization reduces uncertainty inherent in pharmaceutical development. While you cannot eliminate all risks, generative AI helps you identify and address potential issues earlier in the development process.
Challenges and Limitations of Generative AI in Pharma
What obstacles might you encounter when implementing generative AI in your pharmaceutical operations? Understanding these challenges helps you prepare effective mitigation strategies.
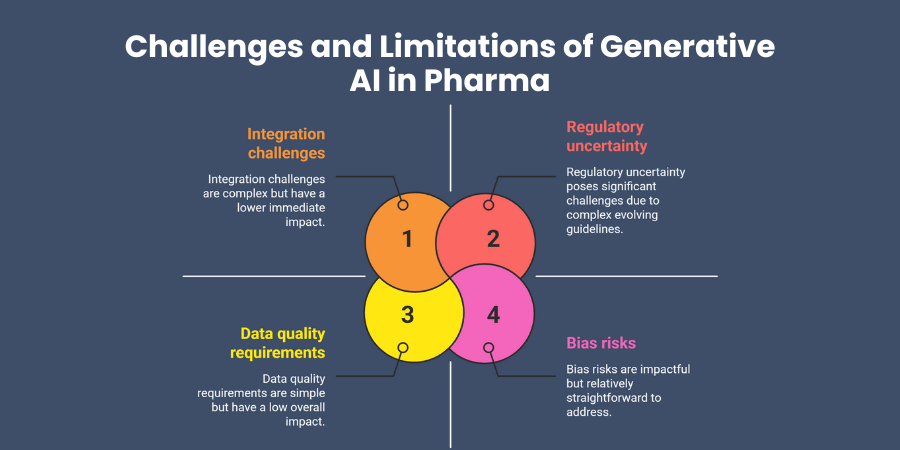
Data quality requirements present immediate implementation challenges. Generative AI models require large, high-quality datasets to function effectively. If your historical data contains gaps, inconsistencies, or biases, the generated outputs will reflect these limitations.
Model explainability concerns affect regulatory acceptance and scientific confidence. When generative AI suggests a novel compound or trial design, you need to understand the reasoning behind these recommendations. Black-box models that cannot explain their decisions create compliance and validation challenges.
Validation complexity increases with generative applications. Unlike traditional analytics that analyze existing data, generative AI creates new content that requires novel validation approaches. You must develop frameworks to verify AI-generated compounds, protocols, and documents.
Regulatory uncertainty surrounds AI-generated content in pharmaceutical applications. While regulatory agencies increasingly accept AI tools, specific guidelines for generative applications remain evolving. You may face longer review times or additional documentation requirements.
Bias risks emerge from training data limitations or algorithmic design choices. If your training datasets underrepresent certain patient populations or therapeutic approaches, the generative models may perpetuate these biases in their outputs.
Integration challenges arise when connecting generative AI systems with existing pharmaceutical workflows, laboratory equipment, and regulatory processes. Legacy systems may require significant modifications to accommodate AI-generated inputs.
Regulatory and Ethical Considerations
How do you ensure your generative AI implementations comply with pharmaceutical regulations and ethical standards? This critical aspect requires careful planning and ongoing attention.
Regulatory compliance frameworks must address AI-specific requirements while maintaining traditional pharmaceutical standards. You need documented validation procedures, audit trails, and quality management systems that encompass AI-generated content.
Data privacy protection becomes more complex with generative models that can potentially recreate or infer sensitive information from training data. Your privacy frameworks must address both input data protection and output data security.
Transparency requirements demand clear documentation of AI model development, training data sources, validation procedures, and decision-making processes. Regulatory agencies expect comprehensive explanations of how AI systems contribute to pharmaceutical decisions.
Human oversight mechanisms ensure that AI recommendations receive appropriate scientific review before implementation. You must maintain clear boundaries between AI assistance and human decision-making authority.
Ethical research standards apply to AI-generated hypotheses, trial designs, and treatment recommendations. Your ethics committees should review AI applications to ensure they align with patient welfare and scientific integrity principles.
How to Implement Generative AI in Pharmaceutical Organizations
Are you ready to begin your generative AI implementation journey? Success requires systematic planning and phased execution that aligns with your organizational capabilities and strategic objectives.
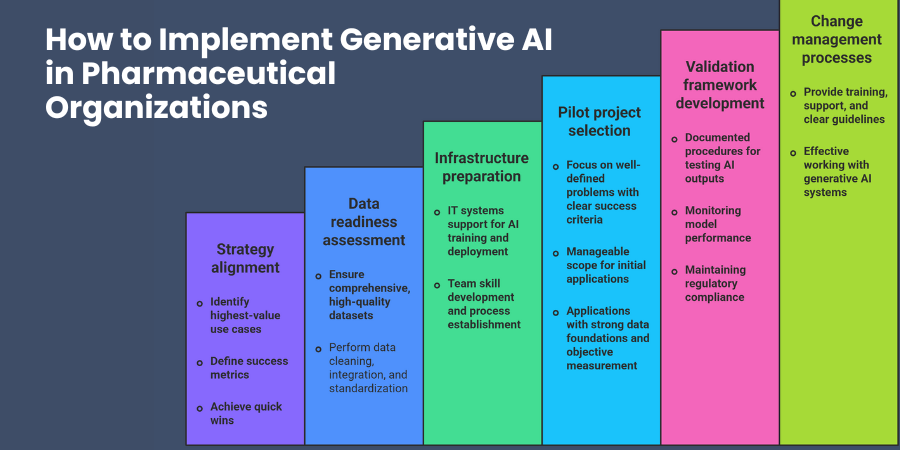
Strategy alignment starts with clear identification of your highest-value use cases and success metrics. Which pharmaceutical processes would benefit most from generative AI? Where can you achieve quick wins that demonstrate value and build organizational confidence?
Data readiness assessment determines your implementation timeline and approach. You need comprehensive, high-quality datasets to train effective generative models. This may require data cleaning, integration, and standardization efforts before AI development begins.
Infrastructure preparation involves both technical and organizational components. Your IT systems must support AI model training and deployment while your teams develop necessary skills and processes.
Pilot project selection should focus on well-defined problems with clear success criteria and manageable scope. Start with applications where you have strong data foundations and can measure results objectively.
Validation framework development ensures your AI implementations meet pharmaceutical quality standards. You need documented procedures for testing AI-generated outputs, monitoring model performance, and maintaining regulatory compliance.
Change management processes help your organization adapt to AI-augmented workflows. Your teams need training, support, and clear guidelines for working effectively with generative AI systems.
Future Trends of Generative AI in Pharma
What exciting developments can you expect in generative AI applications for pharmaceutical innovation? The technology continues evolving rapidly with implications for your long-term strategic planning.
Precision medicine advancement through AI-generated personalized treatments represents a major trend. Future systems will create individualized therapeutic approaches based on genetic, clinical, and lifestyle data unique to each patient.
Autonomous research laboratories powered by generative AI will conduct experiments, analyze results, and generate new hypotheses with minimal human intervention. These systems will accelerate discovery cycles and enable 24/7 research operations.
Collaborative drug discovery platforms will connect pharmaceutical companies, academic institutions, and AI systems in shared research environments. Generative AI in pharma will facilitate knowledge sharing and collaborative innovation across organizational boundaries.
Real-world evidence integration will enhance generative models with continuous learning from patient outcomes, treatment responses, and safety data. This feedback loop will improve AI predictions and recommendations over time.
Regulatory AI assistants will help navigate complex compliance requirements by generating submission documents, predicting regulatory responses, and optimizing approval strategies across multiple jurisdictions.
How Shadhin Lab Can Support Generative AI Adoption in Pharma
Are you looking for expert partners to accelerate your generative AI implementation in pharmaceutical operations? Shadhin Lab specializes in developing cutting-edge AI solutions specifically designed for pharmaceutical and healthcare applications.
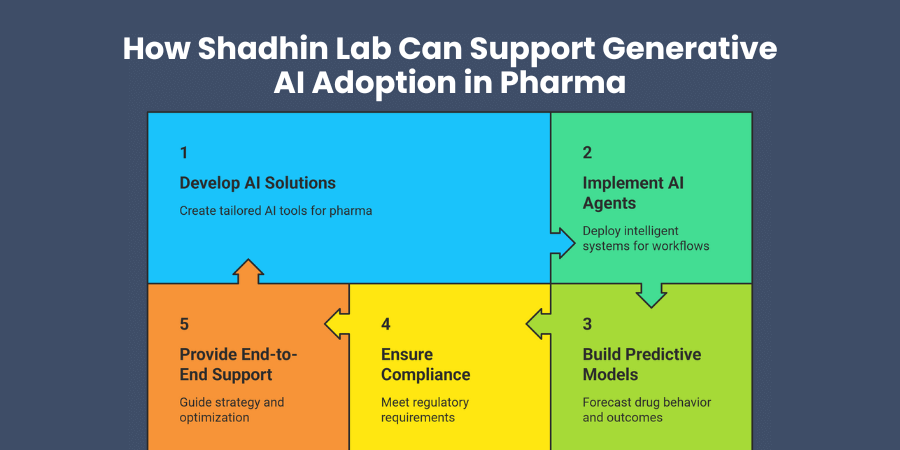
Our generative AI development capabilities encompass the full spectrum of pharmaceutical use cases, from molecular design and drug discovery to clinical trial optimization and regulatory documentation. We understand the unique requirements, compliance standards, and validation needs of pharmaceutical organizations.
AI agent development represents one of our core strengths, creating intelligent systems that can autonomously handle complex pharmaceutical workflows while maintaining human oversight and control. These agents can manage research processes, optimize operations, and generate insights that accelerate your innovation cycles.
Predictive modeling expertise helps you forecast drug behavior, clinical outcomes, and market responses with unprecedented accuracy. Our models integrate multiple data sources and generate actionable predictions that improve decision-making throughout your development pipeline.
Compliance-ready system design ensures your AI implementations meet pharmaceutical regulatory requirements from day one. We build validation frameworks, audit trails, and quality management systems that satisfy regulatory expectations while maximizing AI capabilities.
End-to-end implementation support covers strategy development, pilot project execution, scaling strategies, and ongoing optimization. Our team works closely with your organization to ensure successful AI adoption that delivers measurable business value.
Conclusion
You now have a comprehensive understanding of how generative AI in pharma can transform your organization’s approach to drug discovery, development, and commercialization. The technology offers unprecedented opportunities to accelerate innovation, reduce costs, and improve patient outcomes.
The question is not whether generative AI will reshape pharmaceutical innovation, but how quickly your organization will embrace these capabilities to gain competitive advantage. Companies that thoughtfully implement generative AI strategies today will lead tomorrow’s pharmaceutical breakthroughs.
Your next steps should focus on identifying high-value pilot applications, building necessary data foundations, and developing organizational capabilities for AI adoption. Start small, measure results carefully, and scale successful implementations systematically.
Remember that generative AI augments rather than replaces human expertise. Your scientific knowledge, regulatory experience, and patient focus remain essential for successful pharmaceutical innovation. AI simply provides powerful tools that amplify your capabilities and accelerate your impact.
The future of pharmaceutical development belongs to organizations that successfully combine human insight with artificial intelligence capabilities. Are you ready to begin this transformative journey?
Frequently Asked Questions
How long does it take to implement generative AI in pharmaceutical operations?
Implementation timelines vary significantly based on your data readiness, use case complexity, and organizational capabilities. Simple applications like document generation can be deployed in 3-6 months, while comprehensive drug discovery systems may require 12-18 months for full implementation.
What are the typical costs associated with generative AI adoption in pharma?
Costs depend on implementation scope, data requirements, and infrastructure needs. Initial pilot projects typically range from $100,000 to $500,000, while enterprise-wide implementations can require $1-5 million investments. However, successful implementations often generate 3-5x ROI within two years.
How do regulatory agencies view generative AI applications in pharmaceutical development?
Regulatory acceptance is growing as agencies develop AI-specific guidance. The FDA, EMA, and other authorities increasingly recognize AI tools when properly validated and documented. Key requirements include transparency, validation evidence, and maintained human oversight of AI-generated recommendations.
Can generative AI replace traditional pharmaceutical research methods?
Generative AI complements rather than replaces traditional research methods. The technology accelerates hypothesis generation, candidate identification, and optimization processes, but human expertise remains essential for validation, interpretation, and strategic decision-making throughout pharmaceutical development.
What data requirements are necessary for successful generative AI implementation?
Successful implementations require large, high-quality datasets relevant to your specific applications. Molecular design needs comprehensive chemical databases, while clinical applications require extensive trial data. Data quality, consistency, and completeness are more important than absolute volume for effective AI training.
Shaif Azad
Related Post
Top AI Development Companies in Wyoming
Are you watching Wyoming transform from a traditional energy state into something much more exciting? The...
Top AI Development Companies in Wisconsin
Are you watching Wisconsin transform into an unexpected AI powerhouse? The Badger State is quietly becoming...
Top AI Development Companies in Utah
Have you been watching Utah’s remarkable transformation into a thriving technology powerhouse? Cities like Salt Lake...